RUSP Nomination
Quick Links
- What is the RUSP?
- Organizing for Sucess
- Expert Advisory Group
- Working Focus Groups … including upcoming meeting dates
- Project Consultant Team
Your Voice is needed!
Workgroups are open to everyone to observe – in fact, we encourage your participation as we want inputs from all parts of the MLD and NBS ecosystems. Those who have previously registered here using a red button and have expressed workgroup interest will automatically be registered. If you have not received specific meeting attendance details by a week before the meeting, please re-register here and we will send you access details. Click to Register for one or more workgroupsUpcoming Meetings and Discussions
| _key | Group (all times are Eastern time) | groupNameShort | Feb20 | Mar20 | Apr20 | May20 | Jun20 | Jul20 | Aug20 | Sep20 | Oct20 | Nov20 | Dec'20 | Jan'21 | Feb'21 | Mar'21 | Apr'21 | May'21 | Jun21 | Jul21 | Aug21 | Sep21 | Oct21 | Nov21 | Dec21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | Expert Advisory Group | EAG | Wed 12th @ 6:00 pm ET | Wed 22nd @ 5:30 pm ET | Wed 24th @ 5:30 pm ET | Wed 29th @ 5:30 pm ET | Sat 3rd @ 9:30 am ET | Wed 4th @ 5:00 pm ET | Wed 2nd @ 5:00 pm ET | Wed 13th @ 5:00 pm ET | Wed 3rd @ 5:00 pm ET | Wed 21st @ 5:00 pm ET | Wed 26th @ 5:00 pm ET | Wed 7th @ 5:00 pm ET | Wed 18th @ 5:00 pm ET | Wed 29th @ 5:00 pm ET | Wed Dec 2nd @ 5:00 pm ET | ||||||||
| 10 | Bioethics | B | Mon 27th @ 3:00 pm ET | Fri 4th @ 11:00 am ET | Fri 9th @ 11:00 am ET | Fri 4th @ 11:00 am ET | Fri 18th @ 11:00 am ET | Fri 30th @ 11:00 am ET | Fri 10th @ 11:00 am ET | ||||||||||||||||
| 11 | Standard of Care – Clinical Care & Research | CCR | Tue 28th @ 12:30 pm ET | Tue Aug 18th @ 12:00 am ET | Thu 17th @ 3:30 pm ET | Tue 13th @ 5:00 pm ET | Tue 17th @ 5:00 pm ET | Tue 21st @ 5:00 pm ET | |||||||||||||||||
| 12 | Education & Outreach | EO | Thu 30th @ 4:00 pm ET | Tue 1st @ 3:00 pm ET | Tue 17th @ 8:00 am ET | Wed 23rd @ 5:00 pm ET | Tue 3rd @ 5:00 pm ET | ||||||||||||||||||
| 13 | Public Health | PH | Tue 28th @ 11:00 am ET | Tue Sep 1st @ 1:00 pm ET | Tue 6th @ 1:00 pm ET | Fri 18th @ 2:00 pm ET | Wed 9th @ 1:00 pm ET | Wed 14th @ 1:00 pm ET | Wed 11th @ 1:00 pm ET | ||||||||||||||||
| 14 | Access, Reimbursement, & Legal | ARL | Wed 29th @ 4:00 pm ET | Wed 9th @ 3:00 pm ET | Wed 2nd @ 3:00 pm ET | Wed 7th @ 1:00 pm ET | Wed 11th @ 3:00 pm ET | ||||||||||||||||||
| 15 | Screening & Validation | SV | Mon 20th @ 5:00 pm ET | Tue 15th @ 4:00 pm ET | Mon 26th @ 1:00 pm ET | Wed 17th @ 4:00 pm ET | Thu 1st @ 11:00 am ET | Wed 23rd @ 11:00 am ET | Thu 5th @ 11:00 am ET | ||||||||||||||||
| 16 | Emerging Therapies & Clinical Care | ETCC | Wed 29th @ 11:00 am ET | Mon 21st @ 1:00 pm ET | Wed 18th @ 1:00 pm ET |
Everything you need to know – in 60 seconds or so …
RUSP - Recommended Uniform Screening Panel
It’s Not All About the RUSP
The focus on the RUSP nomination is not purely about a RUSP Approval, rather it’s about the implementation of screening. As described above, the nomination requires a comprehensive evidence-based summary of everything about the proposed screen, all of which will be beneficial for a successful public health implementation. The RUSP nomination package will show evidence of efficacy and consensus – the keys to MLD screening implementation in public health systems. Click to Help or Be Kept Up to DateOrganizing for Success
Extensive effort has been put into establishing an organizational structure for an efficient, timely, and successful RUSP nomination. A core group of MLD and newborn screening experts, the Expert Advisory Group, has been convened to lead the RUSP Nomination preparation, submission, and approval. They will be assisted by seven different Working Focus Groups, each with a different emphasis and made of up representatives from the broader newborns screening, MLD, patient, and advocacy communities. A Project Consultant Team will be assisting to facilitate, handle logistics, and administer the project. As necessary, experts, consultants, and other resources will be engaged to provide specific short-term guidance and inputs. Details on each of these contributors to the project are provided below.
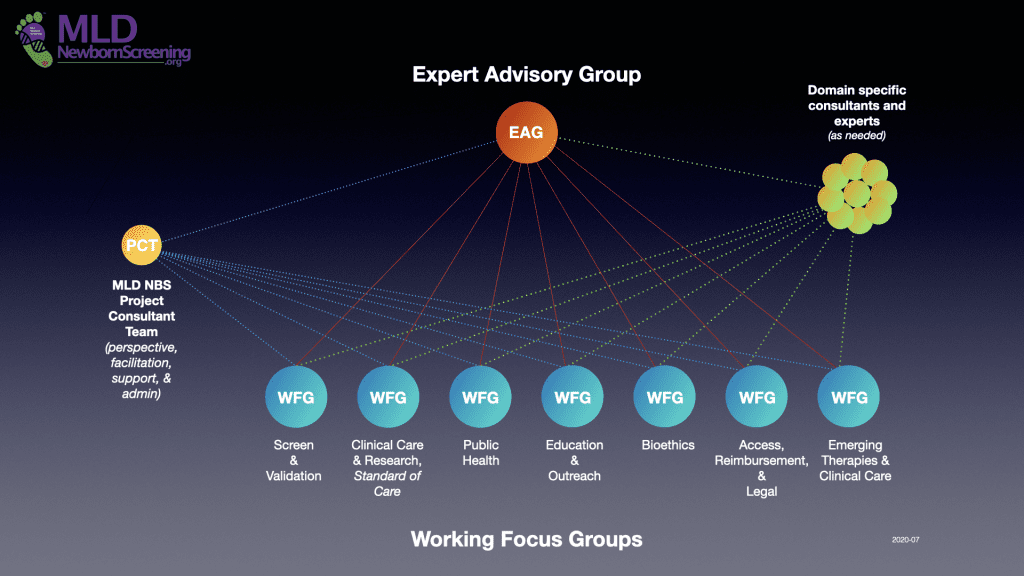
Expert Advisory Group
The EAG was launched in February 2020. They have since started their twice quarterly meetings as they work to guide and manage the MLD RUSP Approval project.
The EAG consists of MLD and newborn screeing experts with a broad perspective and knowledge that covers all of the key components of the RUSP Nomination. Their primary responsibility is to create a complete evidence-based RUSP Nomination.
The EAG will direct the contribution and approach of the Working Focus Groups, and through a healthy interactive dialog identify and develop strategies to address gaps in data, conclusions, publications, and recommendations required by the nomination form.
EAG members:
click on name to expand the bio …
Maria Escolar, MD MS – Director of the Program for the Study of Neurodevelopment in Rare Disorders at Children’s Hospital of Pittsburgh
Amy Gaviglio, MS CGC – Currently under contract with the CDC. Previously worked Minnesota Department of Health, Newborn Screening Program
Joan Keutzer, Ph.D. – Retired in 2019 from Sanofi/Genzyme as Vice President of Scientific Affairs where she was instrumental in Genzyme’s NBS efforts
Joe Orsini, Ph.D. – Deputy Director of the Newborn Screening Program at the New York State Department of NBS lab.
Marc Patterson, MD – Pediatric Neurologist at the Mayo Clinic in Rochester, MN., and is a professor of neurology, pediatrics, and medical genetics.
Melissa Wasserstein, MD MS – Chief, Division of Pediatric Genetic Medicine at Children’s Hospital at Montefiore, and PI for the ScreenPlus NBS pilot study.
Working Focus Groups
The Working Focus Groups (WFG’s) are topic-focused groups guided by, and contributing to, the efforts of MLD NBS Expert Advisory Group. These seven groups will be where extended discussions and insight will be shared in specific topic areas that are critical to a successful RUSP nomination and the implementation of MLD newborn screening.
It is expected these groups will meet at least twice a quarter, generally by video conference, however, there may be some extended time in-person meetings.
Everyone Can Join A WFG!
It’s important to engage and hear from many perspectives as we prepare our RUSP nomination and pursue implementation into various public health programs. Each of you has a unique perspective, experience, and insight to contribute. By including everyone’s inputs we hope to build understanding, perspective, and consensus … all key items as we approach state, province, and national labs for implementation. The Working Focus Groups are where you can directly contribute your thoughts, ideas, and concerns.
I’m Interested – Click to follow or join a WFG
Workgroup Goals
The WFG’s correspond to the primary topics and considerations that are required for the RUSP nomination and to implement MLD NBS screening in public health. (The meeting schedule is at the top of this page)
WFG NAME
AREA of FOCUS
Screen & Validation (S&V)
Chemistry, validation, reliability, and implementation of the lab test. Prof. Gelb will be guiding this group.
Clinical Care & Research (CCR)
This WFG picks up after the family is notified of the screening results and will focus on the diagnostic confirmation, clinical care, and therapy recommendations. Defining long-term feedback of phenotype, clinical care, and therapy results to improve future newborn recommendations. The hope is this group’s work will result in a formal MLD Standard of Care. Dr. Escolar will be guiding this group.
Education & Outreach (E&O)
Education and outreach to NBS screening labs, public health systems, treating clinicians, families, and advocacy in support of the clinical care, therapeutic recommendations and longer-term follow for patient care and to advance research.
Public Health (PH)
Adapt and help implement processes in public health systems, improvement in treating physician notification and guidance for patient notification, diagnostic confirmation, referrals, care management, and follow-up.
Access, Reimbursement, & Legal (ARL)
Access, reimbursement, and other legal & policy issues … in state payor/provider contract implementation, and access across state lines when appropriate therapy is not available locally or regionally.
Forthcoming Clinical Care and Therapies (FCT)
Future care and therapeutics that might impact MLD NBS after the RUSP nomination is submitted, under review, or in the near future.
Bioethics/ELSI (BE)
Ensure the consideration of ethical issues in referral strategies for later-onset disease, clinical trial referrals, strategies when suitable or desired therapy is not accessible, medium & long-term followup, immediate & extended family notifications, etc.. In addition to working with the EAG, the Bioethics WFG will work very closely to inform and guide the other WFGs.
Click to Join a WFG
Project Consultant Team
The Project Consultant Team is an ongoing core support group for the EAG and WFGs throughout the term of the RUSP Nomination and Approval project providing perspective, insight, guidance, and content throughout the project to optimize the efficiency and quality of the preparation, submittal, review, and approval of the MLD RUSP Nomination. The PCT will also provide the infrastructure, a between-meeting collaboration platform, meeting facilitation, and administrative support. They will keep the MLDnewbornScreening.org website up to date as a resource to keep the medical, research, patient, and public health communities informed and to encourage their participation in appropriate areas of the project.
PCT members:
click on name to expand the bio …
Don Bailey, Ph.D. MEd – Director of RTI’s Center for Newborn Screening, Ethics, and Disability Studies and a recent member of the ACHDNC
Tara Mathiesen, BSN – Formerly Senior Director for US Diagnostics at Orchard Therapeutics. responsible for newborn screening and diagnostic partnerships and strategy.
Brad Therrell, Ph.D. – Director of the National Newborn Screening and Global Resource Center. Past President of the International Society for Neonatal Screening (ISNS)
Dean Suhr, BS – Dean is co-founder & President of MLD Foundation, President & Founder of Rare Army, and facilitator of the RUSP Roundtable
MLD Foundation – MLD Foundation is a 20-year-old registered charity serving families globally and take the lead in coordinating the RANSIP NBS project
