MLD Newborn Screening is very complicated – MLD Foundation has launched and is managing a program to deal with and overcome the challenges. 
This article is extracted from a poster first presented at WORLDSymposium in Orlando, Florida on February 12, 2020. You can download the poster here.
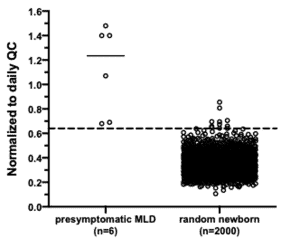
We Have a Working Screen
ScreenPlus - New York Identified Pilot

A consented identified-baby pilot called ScreenPlus (4) will be launching in portions of New York state in the spring of 2020. This study will validate the MLD NBS screen in a state lab, and will provide an opportunity to go beyond in-lab genomic sequencing to make a formal diagnosis, and to refine the screening, diagnostic, clinical and therapeutic recommendation flow.
Pilots are being pursued in other states and countries.
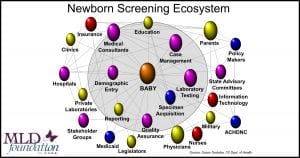
Newborn Screening – It’s Complicated
The NBS ecosystem is very complicated and consists of much more than a lab test. The screen results guide a complicated series of decisions and recommendations for clinical and therapeutic care as well as social services. In the US, NBS can involve both the state and federal governments, insurance companies, caregivers, and many other ecosystem partners in formal and informal roles.
Newborn Screening is Public Health
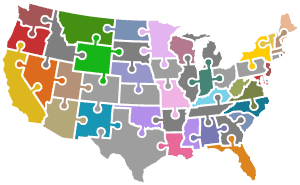
It’s quite a puzzle … each US state – plus the US territories, Washington DC, and the military for total of approximately 56 distinct programs – define their own philosophy, rules, regulations, flows, decision making criteria, processes, legislative oversight, and resources for newborn screening. Also, all of the ecosystem partners identified above have their own role, requirements, and needs that need to be addressed to implement a successful NBS program.
ACHDNC & The RUSP
The federal government does not manage or control state newborn screening, however, the federal Health & Human Services Secretary formally convenes an advisory committee called the ACHDNC (5) that uses an extensive evidence-based review process to maintain the Recommended Uniform Screening Panel (RUSP (6)) which current consists of 35 core conditions. Most states, in a formal or informal manner, reference the RUSP for guidance when prioritizing the consideration and implementation of new screens.

Key MLD Newborn Screening Issues
Beyond the reliability of the screen, a great number of key practical and ethical issues will need to be discussed by key domain leaders and the MLD community as we come to consensus and recommendations for the MLD RUSP nomination. These issues include:
- Clinical and therapeutic care recommendations for late infantile MLD newborns
- What is a viable therapy for MLD newborns
- What if that therapy is not available in your home state (or country)
- Access & reimbursement for expensive therapies
- What should be the role of clinical trials and emerging therapies as therapeutic options
- Can/should juvenile MLD be recommended to be a part of the recommended screen, and if so, what clinical and therapeutic recommendations should be made for suspected juveniles
- Improving genotype/phenotype correlation
- and many more
Next Steps
Building on over a decade of MLD ecosystem experience, 5 years of RUSP Roundtable facilitation, and the 2017 MLD NBS Summit, MLD Foundation is currently facilitating a two-pronged initiative to address the many issues and data points needed for a successful RUSP application.
RUSP Nomination & Approval
The first leg focuses on a RUSP nomination and approval. The launch of a MLD NBS Expert Advisory Group took place on February 12, 2020. The group will work closely with MLD Foundation, other MLD advocacy groups, MLD families, and biopharma partners and will initiate a number of Discussion/Focus/Consensus Group meetings to engage a broad, informed, and collaborative set of interested parties to inform and address the many aspects of the Nomination.
Public Health Implementation
In parallel, MLD Foundation is actively preparing to use the information gathered for the RUSP nomination to formalize strategies to implement MLD NBS in the 56 US public health programs, and internationally.
To be kept update or to engage in various aspects of the MLD RUSP Nomination and screening implementation, please click below:
Click to Engage!
REFERENCES
- Pseudo-deficiency of arylsulfatase A: a common genetic polymorphism with possible disease implications, Hohenschutz, C., Eich, P., Friedl, W. et al. Hum Genet 82, 45–48 (1989). https://doi.org/10.1007/BF00288270
- Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples. Clinical Chemistry 62:1 (2016) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4737087/
- Unpublished and unreviewed preliminary natural history data, Dr. Laura Adang, Children’s Hospital of Philadelphia
- dentifying Rare Diseases in Newborns: Broader Screening for Better Outcomes, Dr. Melissa Wasserstein https://www.montefiore.org/ body.cfm?id=1738&action=detail&ref=1607
- Advisory Committee on Heritable Disorders in Newborns and Children, https://www.hrsa.gov/ advisory-committees/heritable-disorders/index.html
- RUSP – Recommended Uniform Screening Panel, https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html