RANSIP – JULY 2020 EAG LAUNCH UPDATE
This is a brief update on the recent launch of the seven MLD newborn screening Working Focus Groups. Our efforts are inclusive of the MLD, public health, clinical care, and therapeutic ecosystems and are organized under the RUSP Approval and Newborn Screening Implementation Program (RANSIP).
You must sign up and have checked the boxes expressing interest in the WFGs to receive meeting invitations. If you expected but did not receive invitation(s), please click below to express or re-express your interest.
The RANSIP program has two arms … 1) RUSP approval and 2) implementation of the screen globally.
An Expert Advisory Group (EAG) was launched in February of 2020 to guide and facilitate the MLD newborn screening efforts.
The EAG consists of the following individuals (you can learn more about them at the website):
- Maria Escolar, MD MS – Children’s Hospital of Pittsburgh
- Amy Gaviglio, MS CGC – Centers for Disease Control
- Joan Keutzer, Ph.D. – Retired from Sanofi/Genzyme
- Joe Orsini, Ph.D. – New York State Newborn Screening Program
- Marc Patterson, MD – Mayo Clinic, Rochester, MN
- Melissa Wasserstein, MD MS – Children’s Hospital at Montefiore and PI for the ScreenPlus NBS pilot study.
The EAG is supported by a 4-person dedicated Project Consultant Team (PCT) and seven Working Focus Groups (WFG).
The WFGs are listed below along with their next meeting date and time:
- Clinical Care & Research … 12 noon EDT, Tue 8/18
- Public Health … 1 pm EDT, Tue 9/1
- Education & Outreach … 3 pm EDT, Tue 9/1
- Bioethics … 11 am EDT, Fri 9/4 *tentative
- Emerging Therapies & Clinical Care … 1 pm EDT, Wed 9/9
- Access, Reimbursement & Legal … 3 pm EDT, Thu 9/9
- Screening & Validation … 2 pm EDT, Tue 9/15
The Working Focus Group meeting schedule through the end of the year is as follows:
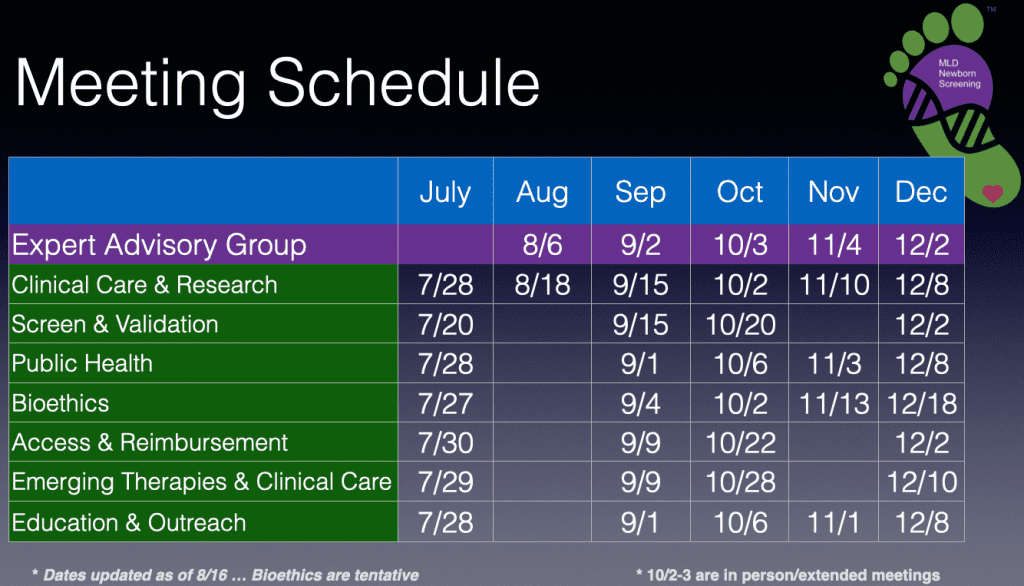
All WFG meetings are open to the public … and you are encouraged to attend either to participate or to observe, however, the EAG meetings are closed and restricted to the EAG members, the PCT, and their invited guests.
WGF Launch Meeting Reports
All seven WFGs launched in July. The overall purpose of the launch meetings was to briefly introduce the RANSIP program and to establish common ground and understanding of the purpose and goals of each WFG.
Some WFGs are on monthly meeting cycles, with some on a 6-week cycle.
Clinical Care & Research WFG
The group agreed on these goals:
- Engage and discuss all perspectives on clinical & therapeutic care
- Assess, develop, and formalize the processes and recommendations from the positive screen through care & therapy including … timing, efficacy, availability, access, follow-up … and alternatives
- Improve how (and why) we make certain recommendations
- Incorporate Ethics (ELSI*) into all aspects of our discussions and strategies
* Ethical, Legal, Social Implications
The discussion touched on several topics …
- Can we demonstrate HSCT as a viable therapy for newborns? Dr. Escolar is preparing a paper describing successful outcomes.
- We discussed the reliability and scope of genotype as a predictor of phenotype. We are going to invite Dr. Adang and others to share some of their data at the September meeting.
- We discussed the importance of an MLD Standard of Care and how the work going into the RUSP care strategy for newborns will form the basis for the broader MLD Standard of Care which can be used for lifetime MLD care guidance, regardless of the mode of diagnostics and the status of disease progression.
We also discussed the impending launch of ScreenPlus in parts of New York State and will respond to a request to review the flow and decision trees ScreenPlus will be using.
The next CC&R EAG meeting is Tuesday, August 18th. We will spend most of our time discussing and starting to build a decision tree and post-screen positive recommendations for families.
Bioethics/ELSI WFG
The group discussed these goals:
- Identify ethical issues related to MLD newborn screening
- Educate, develop guidance, and establish a process to assure ethical considerations are included as the other WFGs and the EAG do their work, have discussions, and make ethically informed decisions on key issues.
The EAG discussed how disparities in access, follow-up, clinical care, confirmatory testing, therapies, finances, other resources, and more, are part of the ethical landscape.
It was agreed that there should be strong interactions with the EAG and other WFGs as the project progresses. It was noted that a decision to not address an issue is indeed a decision.
There was a wide-ranging overview of ethical issues that MLD will be facing including how to ethically recommend therapies across state and national boundaries, across various regulatory status, reporting of juvenile and adult forms, decisions made when there are one or more VoUS mutations, carrier status reporting, reporting of non-MLD findings, access to MLD experts when they cannot be found locally, etc.
It was highlighted that ScreenPlus is a consented project that includes the study of ELSI issues. The WFG members were encouraged to consider what MLD ELSI issues could or should be added to the planned ScreenPlus ELSI studies.
There was some discussion on the group’s interest in highlighting these issues beyond the EAG to the ACHDNC and the overall NBS community as many of the ethical issues MLD is facing and addressing are not unique.
The next meeting is on 9/4. A primary agenda item will be the flows and decision trees CCR WFG and ScreenPlus are developing.
Public Health WFG
The group discussed these goals:
- Establish strong advance communications with public health
- Work together to understand and incorporate the needs of public health into our initial planning, RUSP Nomination, and implementation strategies
- Incorporate ELSI into all aspects of our discussions and strategies … later onset, VoUS, carriers, therapeutic recommendations & access, secondary findings, accessing quality local care, etc.
This WFG is likely evolving to be more of a two-way communication and discussion portal. The public health representatives present were pleased to be invited to the table early to learn about MLD and our screening plans … and to be able to provide feedback, suggestions, and share concerns.
Upcoming meetings will include sharing the Washington State pilot study results, further discussion about the equipment needed, the throughput of the MLD multi-tier screen, and encouragement to have detailed ELSI discussions, including being very sensitive to the difference in the experience of families diagnosed after symptoms to those diagnosed pre-symptomatically.
Screen & Validation WFG
The S&V WFG is focused on implementing and refining the MLD screen in its pilot phase. This WFG has primary responsibility for Part II of the RUSP Nomination.
Prof. Gelb reported that he has submitted a paper reporting on the Washington State MLD deidentified pilot study. The reported data is very stable and consistent. They believe they identified two MLD babies. For the 3rd tier, one had two pathogenic mutations and the other had one pathogenic and two pseudo deficiency mutations.
There was a lengthy discussion about the use of genotype to predict phenotype. In some circumstances, it is a good approach, while in others it is not, yet – or ever?
The EAG discussed the use of the LC column, its availability, and throughput.
The next meeting on 9/15 will continue these discussions and will hopefully include a report on the ScreenPlus pre-launch effort.
Emerging Therapies and Clinical Care WFG
The group discussed and agreed on these goals:
- Address the dynamic nature of emerging therapy(ies) over the anticipated 2-year period during which the RUSP Nomination is prepared and reviewed.
- Create and maintain an addendum to the MLD RUSP Nomination reflecting publicly announced components of emerging therapy and clinical care descriptions, timing, and anticipated targets & efficacy so the ACHDNC, their external Expert Review Group, and the HHS Secretary can best evaluate viable therapy and clinical care for an infant identified through newborn screening.
- Include ELSI in all of our activities … especially with regard to access, alternatives, and family decision-making empowerment.
The ET&CC WFG will not discuss or include comments comparing therapies except by reference to external peer-reviewed publications. Much like the Public Health WFG, this WFG will be a two-way communication and discussion portal.
The industry representatives present were pleased to be invited early to the RUSP Nomination and newborn screening implementation process.
As we all know, the MLD therapeutic landscape is varied and dynamic. When coupled with the multi-year timeframe to prepare and achieve a RUSP nomination, as well as a very long implementation period, the impact of forthcoming therapeutic and clinical advances needs to be part of the shared knowledge.
A brief discussion was held about what we cannot and won’t do in this EAG, including discussing non-public therapeutic status, issues, and performance. Further, there will be no comparison of therapies in this WFG.
The group agreed to prepare guidance for emerging therapies for the RUSP Nomination but will need further guidance, refining, and structure to effectively achieve this goal.
Education & Outreach WFG
This EAG was the last to launch and had a slow start. But that is OK given this group will be primarily responsible to identify the needs and develop strategies using the information and guidance of the other EAGs to address the needs of a changing audience as MLD NBS progresses.
These goals were shared:
- Be a trusted collaborative source for educational and resources in support of families diagnosed with MLD through newborn screening … from diagnosis through clinical and therapeutic decision making.
- Support families, clinicians, public health, (and more targets over time) with compassionate, factual, ethical, and choice-enabling resources
- Connect to our targets in ways that are efficient, practical, and “current” to their style, methods, and expectations
- Support and collaborate with existing NBS educational sources wherever possible
- Include ELSI in all of our activities … especially with regard to access, alternatives, and family empowerment.
The E&O WFG will be working to understand existing repositories and strategies to inform public health, clinicians, and families about MLD and its post-screen recommendations.
Strategic items include addressing the needs of a potential positive screen for ScreenPlus baby #1, as well as the changing target audiences as screening progresses and expands.
The E&O WFG will also support other WFGs as they carry out their responsibilities in access, bioethics, awareness, and of course, family support.
Access & Reimbursement WFG
This EAG will provide input relative to the clinical and therapeutic recommendations relative to channels and barriers that facilitate and limit access to the recommended resources and next steps.
Much of what this EAG will be working on is forward-looking as they prepare for more demand for MLD care and therapeutic resources, most of which will not be available locally.
These goals were shared:
- Identify and proactively address access, reimbursement, and legal issues associated with obtaining optimal clinical care and therapies for families with MLD newborns around the globe.
- Given the great diversity in demographic, geographic, socio-economic, cultural, governmental, and other factors the AR&L WFG will strive to provide awareness, education, resources, and where possible direct support to support families across a variety of clinical & therapeutic options, and circumstances.
- Provide insight into overriding regional and federal policies, reimbursement, appropriations, and processes to organizations engaged in addressing such issues.
- Include ELSI in all of our activities … especially with regard to access, alternatives, and family decision-making empowerment.
This WFG will take some time to build its momentum, however, it is also perhaps one of the most critical groups over the long-term. Newborn screening speeds diagnostics and identifies babies and therapies before irreversible progression, but all of this is for naught if the family cannot access the therapies and clinical care.
